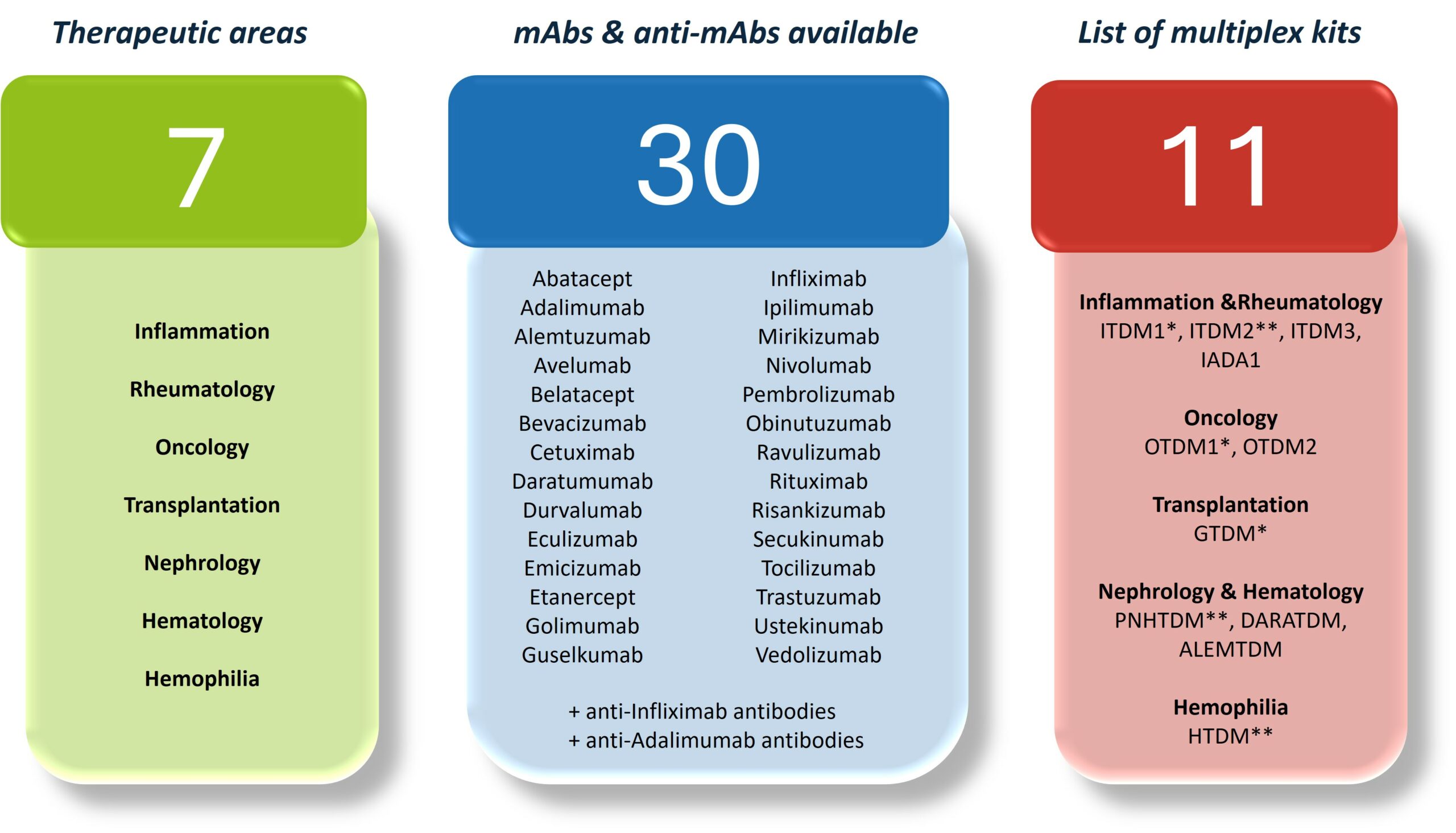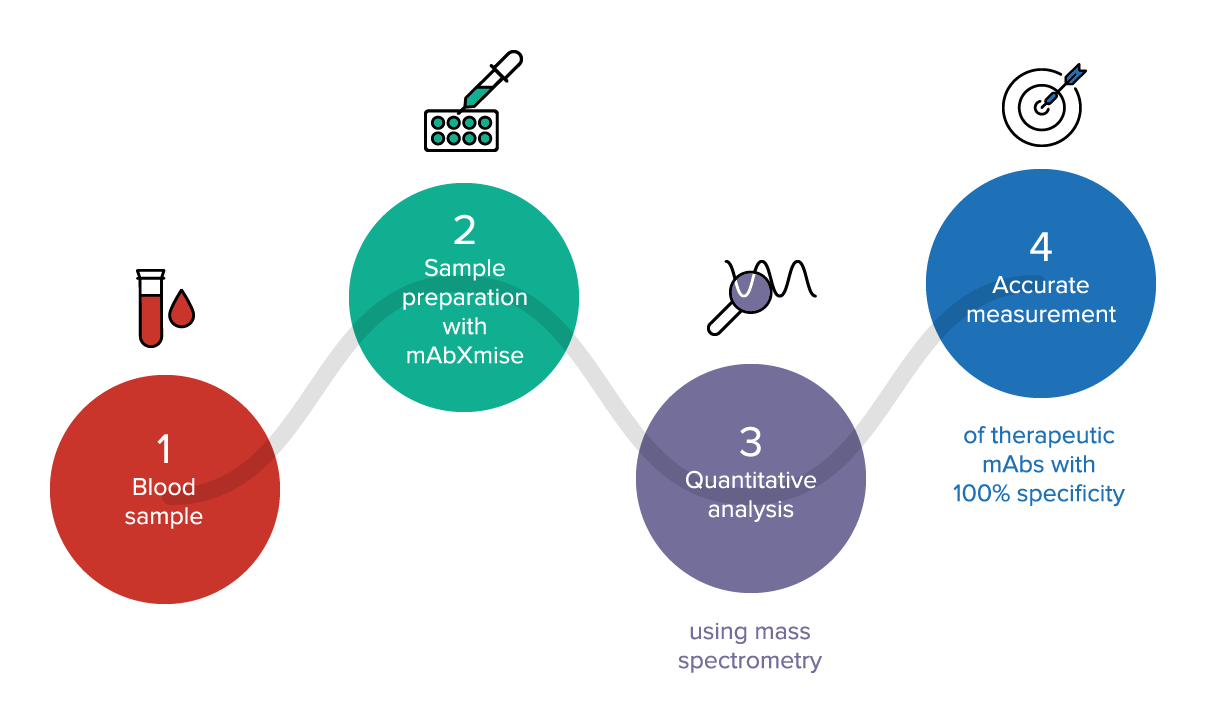
Space reserved for health professionals and professionals using DM-DIV
This space contains promotional content and information on medical devices, the use of which is reserved for healthcare professionals and professionals using DM-DIV. In application of the law of December 29, 2011 relating to the strengthening of health security, I certify that I am a health professional or professional using DM-DIV and therefore be authorized to access this information.